| Table of Contents |  |
|
Review Article
| ||||||
| Fibrates: From PPARα activation to clinical use in the metabolic syndrome | ||||||
| Kate Elizabeth Shipman1, Richard C. Strange2, Sudarshan Ramachandran3,4,5 | ||||||
|
1MRCP, FRCPath, Specialist Registrar in Chemical Pathology, Department of Clinical Biochemistry, University Hospital Birmingham, Birmingham, West Midlands, United Kingdom.
2PhD, FRCPath, Professor of Clinical Biochemistry, Institute for Science and Technology in Medicine, Keele University Medical School, Keele University, Staffordshire, UK, ST5 5BG. 3PhD, FRCPath, Consultant Chemical Pathologist, Department of Clinical Biochemistry, Heart of England NHS Foundation Trust, Birmingham, West Midlands, United Kingdom. 4Professor of Metabolic Medicine, Department of Clinical Biochemistry, University Hospitals of North Midlands NHS Trust, Stoke-on-Trent, Staffordshire ST4 6QG. 5Professor (Hon) of Metabolic Medicine, Faculty of Health Sciences, Staffordshire University, College Road, Stoke on Trent, Staffordshire ST4 2DE. | ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
[Print This Article]
[Similar article in Pumed] [Similar article in Google Scholar] |
| How to cite this article |
| Shipman KE, Strange RC, Ramachandran S. Fibrates: From PPARα activation to clinical use in the metabolic syndrome. Edorium J Biochem 2015;1:8–18. |
|
Abstract
|
|
Pharmaceutical interest in fibric acid derivatives followed the observation in 1953 that administration to rats and humans of compounds derived from dehydrocholic acid resulted in reductions in serum cholesterol levels. It was subsequently shown that these compounds, collectively termed fibrates, effect changes in nuclear transcription leading to a lowering in serum, of triglycerides and low, density lipoprotein cholesterol and elevation of high density lipoprotein cholesterol. However, despite this initial promise large randomized control trials of different fibrates using various primary outcomes have produced discrepant results though sub-group analysis and meta-analysis, do suggest improved benefit in those with atherogenic dyslipidemia. In particular, gemfibrozil trials have shown significant benefit in primary outcomes with other fibrates showing benefit in those with decreased high density lipoprotein cholesterol and raised triglycerides and in secondary outcomes such as diabetic retinopathy. We now after a description of their, actions, review the evidence for fibrate use with a focus on the metabolic syndrome and recommend that their clinical use is targeted at those with reduced high density lipoprotein cholesterol levels and hypertriglyceridemia.
| |
|
Keywords:
Cardiovascular disease risk, Fibrate, High density lipoprotein cholesterol (HDL-C), Metabolic syndrome, Triglycerides
| |
|
Introduction
| ||||||
|
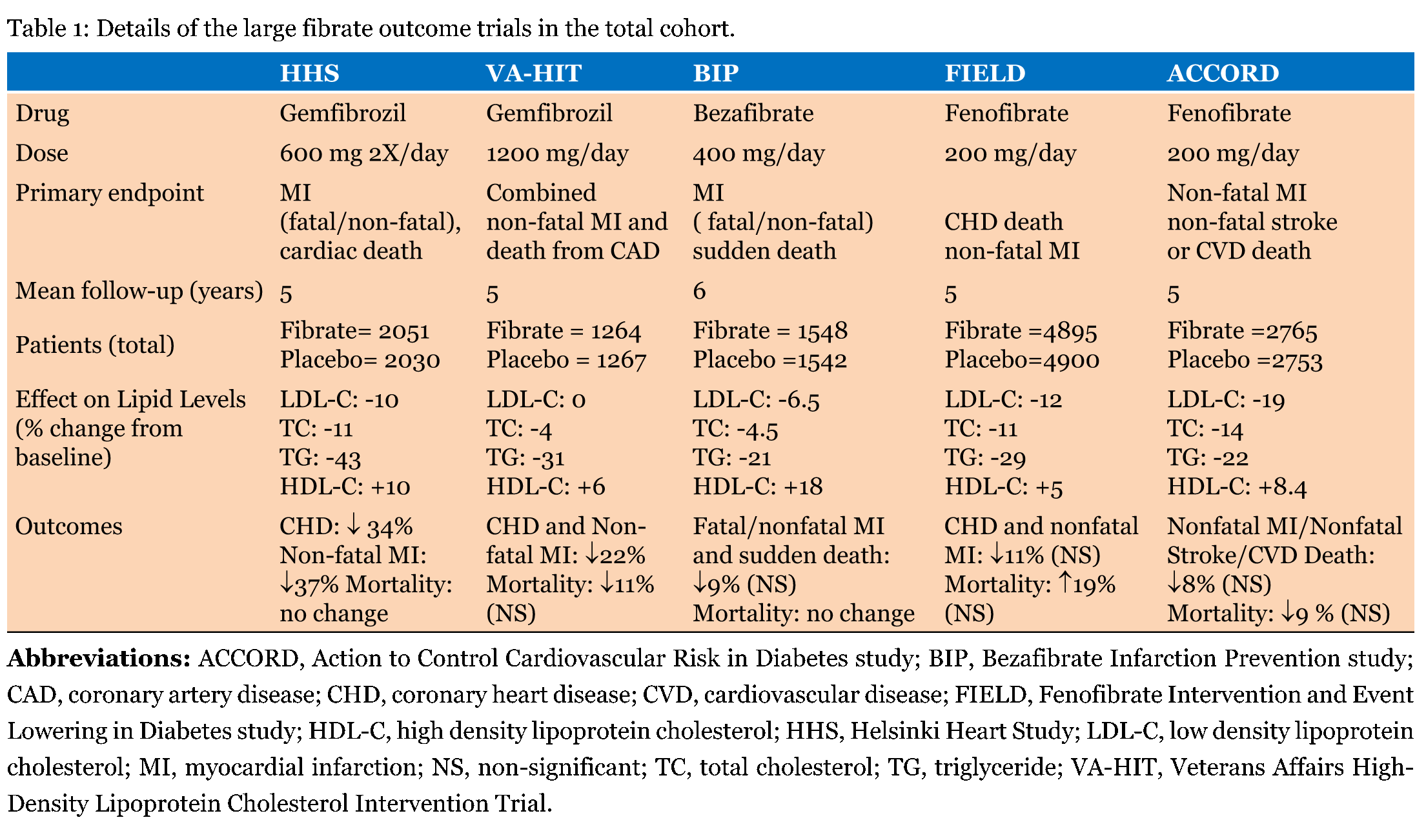
The observation that phenylethyl acetic acid, a derivative of dehydrocholic acid, possessed lipid lowering properties in rats and humans was first made in France in 1953 [1]. Much later the mechanism for this effect was shown to involve nuclear peroxisome proliferator-activated receptors (PPAR). Phenyl ethyl acetic acid originally developed as an insecticide by the Imperial Chemical Industries, caused sickness in farm workers but also demonstrated reduced cholesterol levels [1]. Subsequent work utilizing this unexpected observation has resulted in four potentially useful drugs, clofibrate and fenofibrate (pro-drugs) and gemfibrozil and bezafibrate (active compounds) which variably elevate high density lipoprotein cholesterol (HDL-C) and reduce low density lipoprotein cholesterol (LDL-C) and triglycerides (TG). The evidence supporting their clinical use will be summarized with a particular focus on their use in the metabolic syndrome. PPAR nuclear receptors Cells react to internal and external stimuli in various ways including altering levels of expression of key genes with consequent changes in the levels of appropriate proteins. Maintenance of cellular energy homeostasis is essential and hence, the ability to sense fatty acid/metabolite concentrations in blood is critical. PPARs, first discovered in the early 1990s, are nuclear receptors that mediate relevant gene expression and are key factors in the regulation of metabolism and cellular differentiation. Nuclear receptors are characterized by their modular structure. The domains of PPAR follow the basic nuclear receptor structure and consist of the following; the hypervariable A/B (variable in amino acid sequence and length; for example, this domain encodes 6% and 50% of the entire protein sequence of vitamin D and androgen receptors respectively), the DNA binding C (this part of the nuclear receptor binds to the hormone response element of the gene whose expression it regulates), the hinge D and the ligand binding E domains. The C and E domains remain highly conserved [2]. The nuclear receptors nomenclature committee in 1999 recommended a new phylogenetic based cataloging system with six sub-families based on homology of the DNA and ligand binding domains [3]. There are three subtypes of PPAR genes located on different chromosomes, within the same nuclear receptor subfamily with some common gene sequence: PPARα, PPARγ and PPARß/δ. The degree of expression of the three PPAR genes in tissues varies although co-expression of more than one PPAR in some cells is evident. PPARα (located on chromosome 22q12-q13.1) is principally expressed in hepatocytes, cardiac myocytes, skeletal muscle, proximal renal tubules and enterocytes; all these tissues possess fatty acid oxidation pathways. Expression of the PPARα gene is regulated by various stimuli such as circadian rhythms, stress hormones, leptins released from adipocytes and starvation [4][5]. Thus, hepatic PPARα expression is increased in the starved state. PPARγ (located on chromosome 3p25) is mainly located in adipocytes and the immune system. Interestingly the vitamin D receptor, another nuclear receptor, when activated regulates PPARγ gene expression [6]. The expression of PPARβ/δ is widespread, especially in the brain, adipose tissue and skin. The protein is often found in greater concentrations than the other subtypes, however, the function of PPAR ß/δ is not well characterized [2]. Both the ligands activating the PPAR subtypes and the target genes whose expression are regulated by the PPAR subtypes vary. The PPAR-Retinoid-X-Receptor (RXR) complex, like most nuclear receptors is maintained in an inactive heterodimer state by being bound to co-repressors. When a ligand binds to PPAR, a conformational change in the PPAR-RXR heterodimer leads to dissociation of the inactivating co-repressor. The activated PPAR-RXR heterodimer, following binding of co-activators, interacts with the peroxisome proliferator response element (PPRE) found in the upstream region of target genes [7]. Interestingly, ligand binding to PPAR does not always lead to an activated PPRE complex. The PPRE is also recognized by certain other proteins that can block the function of PPAR. Competition for RXR has been observed with the vitamin D receptor and the thyroid receptor [6] [8]. PPAR can also directly bind factors other than RXR (e.g., thyroid receptor), often forming an inactive heterodimer [9]. PPAR activation also mediates ligand independent phosphorylation. This can affect DNA and ligand binding to the receptor as well as co-activator recruitment. It has been seen that mitogen activated protein kinases, protein kinase A and C, AMP kinase and glycogen synthase kinase-3 alter the activity of PPAR [2]. This could be a possible mechanism by which statins, which are not ligands of PPAR, by inhibiting protein kinase C can activate PPAR leading to anti inflammatory pleiotrophic effects [10] [11]. We later describe unexpected changes in serum lipids following fibrate treatment; no increase in HDL-C in patients also on statins and, in rare instances, a paradoxical reduction in HDL-C. Though the mechanisms of these phenomena have not been elucidated, the complexity of PPAR activation opens up potential clinical possibilities. Actions of FibratesVery low density lipoprotein (VLDL) and LDL particles activate PPARα, which is predominantly expressed in the liver, in the presence of lipoprotein lipase suggesting that esterified triacylglycerols and fatty acids may be natural ligands. Fibrates bind and activate PPARα influencing fatty acid and lipoprotein metabolism in the liver, muscle (both skeletal and cardiac) and kidney leading to a variable increase in serum HDL-C concentrations, a greater reduction in TG levels and a modest decrease in LDL concentrations [12]. Thus, due to elevation of anti-atherogenic HDL and reduction of two atherogenic (LDL and VLDL) particles, it is reasonable to expect that fibrates would have a major role in cardiovascular disease (CVD) management and prevention [13]. While activation of PPARα leads to increased fatty acid oxidation, activation of PPARγ, predominantly expressed in adipose tissue, by its natural (e.g., fatty acids) or therapeutic (thiazolidinediones) ligands, leads to fatty acid storage in the adipocyte, cellular differentiation of the adipocyte, reduction of endothelial inflammation and decreasing insulin resistance [14]. The function of PPARß/δ, expressed in brain, adipose tissue and skin is less well understood. Endogenous ligands for PPARß/δ have not been firmly established, although fatty acids and eicosanoids will activate it [5]. Unlike the other fibrates, bezafibrate will activate all 3 PPAR subtypes [15]. This property may be useful in patients with type 2 diabetes as PPARγ activation could lead to a decrease in insulin resistance. Thus, bezafibrate, but not the other fibrates can affect a decrease in blood glucose levels and glycated hemoglobin and, reduce the incidence of diabetes. In our clinics we have often chosen bezafibrate in place of fenofibrate when glycemic control has been inadequate in diabetic patients with the atherogenic phenotype. Association between CVD and atherogenic dyslipidemiaThe principal action of statins is to competitively inhibit HMG CoA reductase resulting in decreased intracellular hepatic cholesterol synthesis. This leads to increased levels of sterol regulatory element binding protein-2 which in turn results in increased expression of LDL receptors thereby increasing LDL uptake and lowering of serum cholesterol [16]. Following overwhelming evidence of their clinical efficacy, statins have been the pre-eminent lipid lowering agents in most CVD prevention guidelines worldwide [17] [18]. However, despite optimal LDL reduction there remains considerable residual CVD risk, even when other cardiovascular risk factors are taken into account [19]. One possibility is that low HDL and/or raised TG, not sufficiently addressed by statins, may impart some of this risk. These lipid abnormalities may be overlooked once a person has been started on statin therapy [20]. The CVD risk is independently associated with low HDL-C [19]. Elevated TG levels are also linked with CVD [21]. The adverse combination of low HDL-C and elevated TG levels, termed the atherogenic lipoprotein phenotype or atherogenic dyslipidemia, is one of the diagnostic criteria of the metabolic syndrome [22]. Fibrates, via HDL-C elevation and TG reduction would therefore, be expected to be the perfect treatment for atherogenic dyslipidemia and its attendant CVD risk. Fibrate intervention trialsThough epidemiological studies indicate that low HDL-C and elevated TG are associated with CVD risk, the evidence of cardiovascular benefit from fibrate intervention is variable. Unlike intervention studies with statins which have unanimously shown benefit, of the five large randomized control trials (RCTs) using fibrates, the three using fenofibrate or bezafibrate have not shown benefit in their primary outcome measure [23] [24] [25]. Only gemfibrozil has demonstrated clinical usefulness [26] [27]. Importantly, there is marked discrepancy in study outcomes, cohorts and methodologies. Details of the five RCTs are given in Table 1. | ||||||
| ||||||
|
The Helsinki Heart Study (HHS) RCT compared the effects of gemfibrozil (2051 men) with placebo (2030 men) on myocardial infarction and cardiovascular death. The subjects aged 40–55 years and followed up for five years had baseline non HDL-C greater than 5.2 mmol/L (200 mg/dl) [26]. A significant relative risk reduction in cardiovascular outcomes of 34% was observed with 27.3 and 41.4 events per 1,000 individuals in the gemfibrozil and placebo arms respectively. The Veterans Affairs High-Density Lipoprotein Cholesterol Intervention Trial (VA-HIT) in 1999 again investigated the benefits of gemfibrozil versus placebo, in 2531 men with established coronary disease aged under 74 years with HDL-C levels < 1.0 mmol/L (39 mg/dl) [27]. This secondary prevention study is used as primary outcome, myocardial infarction or cardiovascular death. The baseline LDL-C of the cohort was below 3.6 mmol/L (139 mg/dl) to negate LDL-C related risk. A significant relative risk reduction of 22% was associated with the treatment arm (21.7% event rate in the placebo controls against 17.3% in the gemfibrozil treated patients) over a median follow-up of 5.1 years. Lipid changes included elevation of HDL-C (6%) and reduction of TG (31%), but no significant difference in LDL between the groups. These two studies led to a positive outlook for fibrates as a class, though both used gemfibrozil. It appeared that CVD risk associated with HDL-C and TG, both seen as risk factors in population surveys, could be reduced by fibrates; a position of relative clarity at the end of the VA-HIT. A non-significant effect on a composite outcome of myocardial infarction and sudden death was observed in the Bezafibrate Infarction Prevention (BIP) study in 2000. Bezafibrate was compared to placebo in 3090 men and women aged between 45–74 years with established coronary heart disease [15]. Inclusion criteria also included total cholesterol 4.7–6.5 mmol/L (181–251 mg/dl) and HDL-C ≦ 1.17 mmol/L (45 mg/dl) [13]. The study found reduced sudden death and fewer myocardial infarctions (both fatal and non-fatal) in the patients treated with bezafibrate (13.6%) compared with the controls (15%), albeit not significantly. The Fenofibrate Intervention and Event Lowering in Diabetes (FIELD) study investigated the effects of fenofibrate (4895 individuals) versus placebo (4900 individuals) in subjects aged 50–75 years with type 2 diabetes mellitus [23]. The subjects, though not on statin therapy at entry, were given statins to comply with the increasing evidence of benefit and guidelines issued during the trial period [28]. The study cohort comprised 7664 primary and 2131 secondary prevention individuals. Inclusion criteria of the study were total cholesterol 3.9–6.5 mmol/L (151–251 mg/dl), with either a TG/HDL-C ratio ≥ 4.0 or TG between 1.0–5.9 mmol/L (88–522 mg/dl). Patients with hypercreatininaemia (plasma creatinine >130 µmol/l) were not included. Over a mean follow-up of five years, 5.2% of individuals in the fenofibrate arm and 5.9% in the control arm suffered a coronary event, this difference not reaching statistical significance. However, the study was perhaps flawed by statin treatment given post recruitment differing between the trial arms (17% and 8% control and fenofibrate groups respectively). It was accepted this may have masked a larger treatment benefit from fenofibrate. The Action to Control Cardiovascular Risk in Diabetes (ACCORD-LIPID) study was designed to determine the effects of fenofibrate as an add-on agent in individuals with type 2 diabetes mellitus receiving statins [24]. Fenofibrate or placebo was given to 5518 patients on open-label simvastatin with or without established CVD. Glycated Hb was ≥ 7.5% (58 mmol/mol) and, if CVD was present, age at entry was restricted to 40–79 years. When two additional (to diabetes) CVD risk factors were present the age range for inclusion was 55–79 years. The LDL–C at entry was 1.55–4.65 mmol/L (60–180 mg/dl) and HDL–C <1.29 mmol/L (50 mg/dl) for men and <1.42 mmol/L (55 mg/dl) for women. TG levels were <8.5 mmol/L (752 mg/dl) and <4.5 mmol/L (398 mg/dl) for those receiving and not previously receiving lipid therapy respectively. The primary outcome was first occurrence of non-fatal myocardial infarction or stroke or death from cardiovascular causes. There was no significant between-group difference in outcome; 2.4% in the controls and 2.2% in the fenofibrate treated patients over a mean follow-up of 4.7 years [24] [29]. There were no significant differences between groups in the secondary outcomes consisting of the individual components of the primary outcomes and an expanded composite to include hospitalization for heart failure, revascularization and total mortality. Results are therefore, conflicting and the question remains as to whether fibrates have a clinical role. Is there heterogeneity among fibrates with benefit specific to gemfibrozil? This is problematic as adverse effects were more common when gemfibrozil was combined with a statin. Gemfibrozil, unlike other fibrates, does not down-regulate the renal cyclooxygenase enzyme system, this impairing the synthesis of vasodilatory prostaglandins. Though the implications of these findings are not clear, it is important to be aware of within class differences [30]. Subgroup analysis and meta-analysis of fibrate trialsAn important issue was whether, within the heterogeneous groups studied in these trials, there were subgroups of patients who did benefit from fibrate treatment. Sub-group analyses of the RCTs may provide hints as to which patients could benefit without harm, from treatment with a particular fibrate. There are limitations in the interpreting of subgroup analysis data, not least lack of power to detect the heterogeneity of treatment effects and multiplicity [31]. Clearly, another RCT with narrower inclusion criteria might better answer this question. In the Helsinki Heart Study a subgroup analysis of patients with elevated TG and reduced HDL-C showed a greater relative risk reduction than in the remaining cohort [32]. This provided the first indication that the clinical benefits of fibrates may lie in patients with atherogenic dyslipidemia, the lipid profile characterizing the metabolic syndrome. Interestingly, subgroup analysis of the other fibrate trials described above has also suggested that cardiovascular benefits appear to be maximal in subjects with insulin resistance and other characteristics of the metabolic syndrome [33] [34] [35]. Bruckert et al. carried out an analysis of five large trials, selecting trial participants by cut-offs closest to that of atherogenic dyslipidemia (HDL-C <0.91 mmol/L (35 mg/dl) and TG > 2.2 mmol/L (195 mg/dl) [36]. Patients possessing these characteristics accounted for between 11–33% of the total cohort and saw a significant reduction in cardiovascular risk of 28%. Importantly, the complementary group demonstrated only a non-significant 6% reduction in risk [36]. A meta-analysis of the outcome trials with fibrates over the past 40 years or so have been usefully summarized by Jun et al. in 2010 [37]. This analysis considered 18 RCTs using fibrates based on 45,058 individuals; outcomes included major cardiovascular events, coronary events, coronary revascularization, stroke, heart failure, cardiovascular deaths, new-onset albuminuria and adverse effects due to the drugs. Significant decreases were observed in major cardiovascular events (including myocardial infarction and cerebrovascular events). No significant change in mortality, all cause or specific to cardiovascular disease was apparent. They also carried out sub-group analyses demonstrating a greater effect in trials with higher mean TG levels [37]. Fibrates in combination with nicotinic acidNiacin (nicotinic acid) was first shown to reduce serum cholesterol in 1955 [38]. In addition, it led to an increase in HDL-C and a lowering of TG [39]. Nicotinic acid was the first of all lipid lowering agents to demonstrate a positive effect in CVD; the coronary drug project (investigating five treatment regimes) carried out between 1966 and 1975 in males between the ages of 30–64 years following a myocardial infarction, showed that the combination of clofibrate and nicotinic acid lowered non-fatal myocardial infarction by 27% compared with placebo [40]. Nicotinic acid monotherapy was also seen to significantly reduce myocardial infarction by 10%. The atherothrombosis intervention in metabolic syndrome with low HDL/high triglycerides: impact on Global Health (AIM-HIGH) trial enrolled 3,414 participants in the United States and Canada with a history of CVD on statin treatment with low HDL-C and high TG [41]. Participants were in addition to simvastatin, randomly assigned to either placebo (n=1696) or high dose, extended-release niacin (found to have better tolerability than standard nicotinic acid) (n = 1718) in gradually increasing daily doses up to 2,000 mg. It should be noted that ezetimibe, another LDL-C reducing agent was given if the LDL-C target of 40–80 mg/dl (1.03–2.07 mmol/l) was not met. The AIM-HIGH trial was discontinued a year early in 2011 as the CVD event rates in both arms were similar. Similarly, the Heart Protection Study 2 - Treatment of HDL to Reduce the Incidence of Vascular Events (HPS2-THRIVE) study was designed to investigate the benefits of adding niacin (in combination with laropiprant, a prostaglandin D2 antagonist to reduce flushing, a common side effect of niacin) to statin treatment in 25,673 patients with vascular disease [42]. The addition of niacin/laropiprant was not seen to decrease vascular events. However, adverse events were significantly greater in the niacin/laropiprant arm; worsening glycaemic control, incidence of diabetes, bleeding, infections, adverse effects of gastrointestinal, musculoskeletal systems and skin related events. This led to the withdrawal of the drug in Europe. Thus, as in the fibrate case, adding niacin to statins did not reduce CVD. It would however, be interesting examine the benefits of fibrate/niacin therapy in patients not on statins considering the positive outcome seen in the coronary drug project [40]. Recently, Gomaraschi et al. investigated the effects of fenofibrate and niacin in 37 patients with the metabolic syndrome recruited from a multicentre, randomized, open-label, cross-over study [43]. Fenofibrate or extended release niacin not only increased HDL-C levels but also improved the endothelial protective effects of HDL. Treatment with both fenofibrate and extended release niacin increased the ability of HDL to inhibit TNFa-induced vascular cell adhesion molecule-expression and both also improved the impaired HDL ability to induce the expression of endothelial nitric oxide (NO) synthase and NO production. Interestingly, HDL isolated after treatment showed an ability to promote endothelial NO release similar to HDL isolated from controls. With both drugs, HDL function was improved irrespective of baseline HDL-C levels. This once again suggests that further study of not just fibrates, but also other drugs such as extended release niacin may offer new avenues in the treatment of the metabolic syndrome. Effects of fibrates on microvascular complicationsThere has been considerable interest in the ability of fenofibrate to reduce the rates of microvascular complications. The FIELD study demonstrated that fenofibrate significantly reduced the rate of progression of albuminuria [23]. More patients in the treatment arm did not progress, or even regressed, compared to the control arm. The FIELD wash out sub-study also showed less progression of albuminuria, with more patients not deteriorating or even regressing after five years of washout [44]. Lower creatinine values were observed in patients who were on fenofibrate during the trial. However, importantly the reduction in albuminuria and the increased number of patients whose albuminuria did not progress or regressed was not observed in the FIELD patients recruited in Helsinki; this may have been due to lower patient numbers [45]. The ACCORD-LIPID trial also showed that adding fenofibrate to simvastatin was associated with significantly reduced development of both microalbuminuria and macroalbuminuria [29]. FIELD also showed that significantly fewer patients required laser treatment for either macular edema or proliferative retinopathy [46]. In the ACCORD Eye study, progression of diabetic retinopathy was also reduced by intensive glycemic control and addition of fenofibrate to simvastatin. However, no improvement in blood pressure was noted [24]. Importantly, the above microvascular complication rates were not included as primary end points [29]. Thus, we urgently require studies with microvascular complications as primary end-points to help determine the baseline characteristics of those patients who may experience maximum benefit. Interesting observations following fibrate treatment in a non-trial settingInconsistent results with regards to cardiovascular outcomes have been a common finding in fibrate RCTs. Subgroup analysis of even the negative trials suggests significant reduction in CVD in patients with low HDL-C and high TG but the HDL-C change varied in these trials. Our studies in patients in metabolic clinics at the Heart of England Foundation Trust have shown that HDL-C change following fibrate treatment is independently associated with pre-treatment HDL-C levels, diabetes and treatment duration [47]. On stratification, patients with a baseline HDL-C < 1.0 mmol/L (39 mg/dl) showed a significantly greater HDL-C increase compared to patients with a baseline HDL-C ≥ 1.0 mmol/L (39 mg/dl). The relationship was not observed in patients already on statins. Our findings may explain the benefit observed in patients with low HDL-C in subgroup analyses of the fibrate RCTs, and we speculated that fibrates should be reserved for these patients. Further, it is interesting to determine whether adding statins could negate the effects of fibrates and contribute to the outcomes seen in the FIELD and ACCORD-LIPID trials [23] [29]. As we have seen previously statins can activate PPARα directly. Thus, it would be interesting to compare change in PPARα expression before and after addition of a statin to patients on different fibrates. The relationship between HDL-C change and pre-treatment levels was previously hinted by Kornitzer et al. who reported in a study of 1334 patients, that increases in HDL-C levels following at least six months fenofibrate treatment were greatest in patients with lower pre-treatment HDL-C [48]. While a significant 15.2% mean increase in HDL-C in the total cohort was observed, patients with baseline HDL-C ≦ 0.91 mmol/L (35 mg/dl) demonstrated a much larger increase of 37.9%. We also observed that TG change was associated with pre-treatment levels of TG; the higher the pre-treatment level the greater the reduction [49]. However, TG reduction was independent of the baseline HDL-C and seen in all subgroups including patients on statins. The analysis suggested that there was a divergence in the mechanisms affecting HDL-C and TG concentrations following fibrate treatment. The HDL-C increase described above has been observed in clinical practice and trials. However, a rare phenomenon, termed "paradoxical HDL-C decrease" has been described in a few patients treated with fibrates and the closely related thiazolidinediones (PPAR? agonists). For example, a paradoxical HDL decrease was reported in a case series of five patients following fibrate and rosiglitazone treatment [50]. We were the first to observe that pioglitazone also showed this phenomenon [51]. Keidar et al. estimated this paradoxical change to be found in 0.02% and 1.39% of patients treated with fibrates and a combination of fibrates and glitazones respectively [52]. The mechanism of this phenomenon has not been elucidated and it may prove to be difficult to unravel due to its low incidence. Our view is that the mechanism is PPARα based as following thiazolidinediones, patients demonstrated improvement in glycaemia control and PPARγactivators have been shown to alter HDL metabolism via the PPARα receptor [53]. We have also published the only known case of paradoxical HDL-C reduction (classified as > 50% reduction in HDL-C) following statin treatment [54]. A decrease in HDL-C (from 1.8–0.6 mmol/l, 69.6–23.2 mg/dl) was observed following simvastatin and atorvastatin, but not pravastatin or rosuvastatin treatment. Although differences in pharmacokinetics do exist between the statins no ready explanation is available as LDL-C reduction was seen with all statins; the scale of reduction in accordance with the efficacy of the drug and dosage. Once again due to the rarity of the paradoxical HDL-C decrease, we have no data regarding long-term outcome with or without fibrates. As statins interact with PPARα, we can speculate again that the paradoxical HDL-C decrease is PPARα based. A recent study by Ota et al. reported on HDL-C decrease following the initiation of statin treatment following an acute myocardial infarction [20]. A decrease in HDL-C (44–40 mg/dl, 1.14–1.03 mmol/l) was observed in 104 of the 724 patients (follow-up: 218 ± 61 months). This contrasted with an increase in HDL–C (37–48 mg/dl, 0.96–1.24 mmol/l) in the remaining 620 patients (follow-up: 236 ± 111 months). It was seen that a composite of all cause death, myocardial infarction and stroke was significantly higher in the group where HDL-C fell (hazard ratio: 1.95, 95% confidence intervals: 1.08–3.52) despite comparable post-treatment LDL-C levels. Although the outcome of the study was interesting it should be noted that other factors such as weight loss, physical activity, smoking status and general health that could alter HDL-C levels were not recorded. However, this study does indicate that more work needs be carried out on HDL-C, both concentrations and efflux of cholesterol via the HDL particle. Non-alcoholic steatohepatitis/non-alcoholic fatty liver disease (NASH/NAFLD) is associated with the metabolic syndrome with many patients requiring fibrates. We have reported improved liver function tests following treatment with fibrates with maximal decreases seen in patients with higher baseline ?-glutamyl transferase, alanine amino transferase and alkaline phosphatase activities [55]. We speculated that this change in liver function tests is associated with improvements in NAFLD/NASH because it reflects decreased accumulation of hepatic fat and subsequent inflammation. However, no liver biopsies were performed in this cohort to confirm this suggestion. The current role of fibrates in the treatment of dyslipidemiaLipid modification guidelines (CG181) from the National Institute for Health and Care Excellence (NICE) in the UK in 2014 suggest that fibrates should not be routinely offered for CVD prevention to individuals treated for primary or secondary prevention, chronic kidney disease or with type 1 diabetes mellitus [17]. The same guidelines also state that urgent referral to a specialist is required when TG concentrations are above 20 mmol/L (1772 mg/dl) and not due to excessive alcohol or poor glycemic control. Repeat TG levels 5–14 days later, are recommended if found to be between 10–20 mmol/L (886–1772 mg/dl) when fasted and that the patient be referred if the levels remain > 10 mmol/l. Management recommendations are suggested if TG are 4.5–9.9 mmol/L (399–877 mg/dl) though none of the interventions are very specific and they ignore the underlying differences in their action on lipoprotein metabolism. The European Society of Cardiology/European Atherosclerosis Society guidelines focus more on individual care based on disease mechanisms and evidence [18]. Fibrate therapy is recommended for consideration in patients with mixed dyslipidemia and hypertriglyceridemia. Our view is that this takes into account available evidence and lipoprotein science. Rightly, NICE do emphasize that decisions must be made by practitioners based on available evidence and lipid results rather just guidelines. The future for fibrates: Should they be used mainly in the metabolic syndrome?Fibrates exert their effects via complex mechanisms. Heterogeneity of response has been observed which may account for the varied outcomes observed in RCTs. Beneficial changes in lipoprotein metabolism are seen following PPARα agonism by fibrates. Subgroup analysis of the trials suggests that fibrates should be considered in patients with low HDL-C and elevated TG levels. This lipid pattern, atherogenic dyslipidemia, is a classifying feature of the metabolic syndrome, a combination of hypertension, dyslipidemia and glucose intolerance historically known as syndrome X [22] and is associated with significant residual cardiovascular risk even following statin treatment. The classification is presented in Table 2. Thus, we speculate that fibrate therapy should be largely restricted to this cohort. It is important to recognize that the diagnosis of metabolic syndrome does not add any extra risk of CVD, rather the cardiovascular risk associated with the syndrome is the sum of the individual classifying components. For example, in a review of 3459 patients with plaque progression as outcome, while the metabolic syndrome was significantly associated with increased plaque, the relationship lost significance when adjusted for serum TG, body mass index, HDL-C, blood pressure/treatment of hypertension [56]. In the multiple regression model only serum TG ≥150 mg/dl (1.7 mmol/l) remained significantly associated with plaque progression [56]. However, while the metabolic syndrome does not add prognostic risk to an individual, we argue that it makes it easier to adopt a holistic approach to patient care rather than a focus on individual risk factors. Thus, when mechanisms are studied the entire network of risk factors is considered. Other risk factors associated with the metabolic syndrome that require further study are the hormones 1, 25-dihydroxyvitamin D and testosterone [57] [58] [59]. Low levels of both hormones have been associated with the metabolic syndrome. Low vitamin A, zinc and magnesium are also associated with the metabolic syndrome [60] [61][62]. Some studies have suggested that these nutrients are associated with the functions of vitamin D [63] [64] [65] [66] [67]. Hence, interactions between these nutrients and vitamin D need to be studied in patients with the metabolic syndrome. The impact of replacement therapy (vitamins A, D, zinc and magnesium) on the classifying factors of the metabolic syndrome is less clear. When further work is carried out on fibrates it would be useful to consider the influence of these factors on the heterogeneity of response we have described. | ||||||
| ||||||
|
Conclusion
| ||||||
|
While there is overwhelming evidence supporting statins as primary lipid lowering agents, this should not be mistaken for exclusivity. A residual risk of macrovascular disease remains after statin therapy in patients with atherogenic dyslipidemia. There is sufficient supporting evidence for fibrates to be considered in this type of dyslipidemia where statins may have limited efficacy. Further, microvascular benefit was also seen in individuals with type 2 diabetes mellitus, which is a component of the metabolic syndrome. Thus, guidelines, such as those published by NICE in the United Kingdom may not have addressed cardiovascular risk reduction completely. As acknowledged by NICE, guidelines are to guide clinicians, not to override decisions appropriate to an individual patient. Thus, an understanding of the metabolic syndrome as well the actions of fibrates may lead to significant health benefits. Metabolic syndrome is prevalent and on the increase and even a small lowering of risk with fibrate therapy should yield significant benefits. Higher potency PPARα agonists are in development that may produce greater lipid level modification in those already on statin therapy which may help to clarify a role for fibrates in cardiovascular risk modification. | ||||||
|
References
| ||||||
| ||||||
|
[HTML Abstract]
[PDF Full Text]
|
|
Author Contributions
Kate Elizabeth Shipman – Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published Richard C Strange – Analysis and interpretation of data, Revising it critically for important intellectual content, Final approval of the version to be published Sudarshan Ramachandran – Substantial contributions to conception and design, Acquisition of data, Analysis and interpretation of data, Drafting the article, Revising it critically for important intellectual content, Final approval of the version to be published |
|
Guarantor of submission
The corresponding author is the guarantor of submission. |
|
Source of support
None |
|
Conflict of interest
Authors declare no conflict of interest. |
|
Copyright
© 2015 Kate Elizabeth Shipman et al. This article is distributed under the terms of Creative Commons Attribution License which permits unrestricted use, distribution and reproduction in any medium provided the original author(s) and original publisher are properly credited. Please see the copyright policy on the journal website for more information. |
|
|